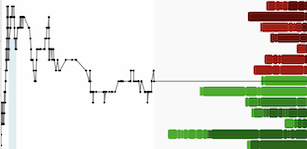
Immunome, Inc. (Nasdaq: IMNM), a biotechnology company focused on developing first-in-class and best-in-class targeted cancer therapies, recently presented preclinical data showing its proprietary antibody-drug conjugate (ADC) payload HC74 overcomes multiple mechanisms of ADC resistance, including payload efflux and target heterogeneity.
HC74 is a novel topoisomerase I inhibitor that serves as the payload in IM-1021, a ROR1-targeted ADC currently in a Phase 1 trial for solid and liquid tumors, and as the payload in several preclinical candidates within the Immunome pipeline.
The data were presented on Oct. 23, 2025, in a poster entitled “HC74, a novel topoisomerase I inhibitor payload for antibody-drug conjugates that overcomes multi-drug resistance” at the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics in Boston.
Highlights of the poster include:
- Over-expression of drug efflux transporters such as ABCC1 and ABCB1 drives primary and acquired resistance to approved ADC payloads and standard chemotherapies but not to HC74
- HC74 exhibits high membrane permeability, leading to enhanced cytotoxicity and robust bystander activity
-
ADCs incorporating HC74 show meaningful efficacy in multiple preclinical tumor models, including:
- Colorectal cancer refractory to trastuzumab-DXd and irinotecan
- Models with acquired resistance to trastuzumab-DXd
- Non-small cell lung cancer with heterogenous target expression
“HC74 is designed to overcome key limitations of existing ADC payloads,” said Immunome’s Chief Scientific Officer, Jack Higgins, Ph.D. “Clinical data show high levels of efflux transporters and target heterogeneity can reduce ADC efficacy. We believe HC74’s ability to overcome those resistance mechanisms supports its potential as a best-in-class payload. We look forward to advancing IM-1021 and our broader HC74 pipeline.”
A copy of the poster is available in the “Events & Presentations” portion of Immunome’s website.
About Immunome, Inc.
Immunome is a clinical-stage targeted oncology company committed to developing first-in-class and best-in-class targeted therapies designed to improve outcomes for cancer patients. We are advancing an innovative portfolio of therapeutics, drawing on leadership that previously played key roles in the design, development, and commercialization of cutting-edge targeted cancer therapies, including antibody-drug conjugate therapies (ADCs). Our most advanced pipeline programs are varegacestat (formerly AL102), a gamma secretase inhibitor which is currently in a Phase 3 trial for treatment of desmoid tumors; IM-1021, a ROR1-targeted ADC which is currently in a Phase 1 trial; and IM-3050, a FAP-targeted radioligand, which recently received IND clearance. Our pipeline also includes IM-1617, IM-1335, and IM-1340, all of which are preclinical ADCs pursuing undisclosed targets with expression in multiple solid tumors. For more information, visit www.immunome.com.
Cautionary Statement Regarding Forward-Looking Statements
Statements in this press release that are not purely historical in nature are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. We use words such as “can,” “expected,” “expects,” “believe,” “promising,” “potential,” “look forward” and similar expressions to identify these forward-looking statements. These forward-looking statements include, but are not limited to, the potential benefits and drug profile of IM-1021 and HC74, including HC74’s potential to overcome resistance mechanisms and potential to become a best-in-class payload; Immunome’s intention to advance IM-1021 and its broader HC74 pipeline; and other statements regarding management’s intentions, plans, beliefs, expectations or forecasts for the future. These forward-looking statements are based on Immunome’s current expectations and involve assumptions that may never materialize or may prove to be incorrect; consequently, actual results may differ materially from those expressed or implied in the statements due to a number of factors, including, but not limited to, the risk that Immunome will not be able to realize the benefits of its strategic transactions; the risk that regulatory approvals for Immunome’s product candidates are not obtained, are delayed or are subject to unanticipated conditions; the risk that pre-clinical data may not be predictive of clinical data; the risk that Immunome’s product candidates fail to achieve their intended endpoints; and other risks and uncertainties indicated from time to time described in Immunome’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2025, filed with the SEC on August 6, 2025, and in Immunome’s other filings with the SEC. Except as required by law, Immunome assumes no obligation and does not intend to update any forward-looking statements included in this press release.
View source version on businesswire.com: https://www.businesswire.com/news/home/20251023619001/en/